Mammography Level 2 Violations
In March 2018, the US FDA implemented a rule called “Enhancing Quality Using the Inspection Program” (EQUIP), which added new, important requirements for mammography providers beyond those of the original Mammography Quality MQSA. Compliance with EQUIP requirements was to be assessed during each MQSA inspection. The consequence of failing to meet EQUIP requirements could result in a Level 2 violation.
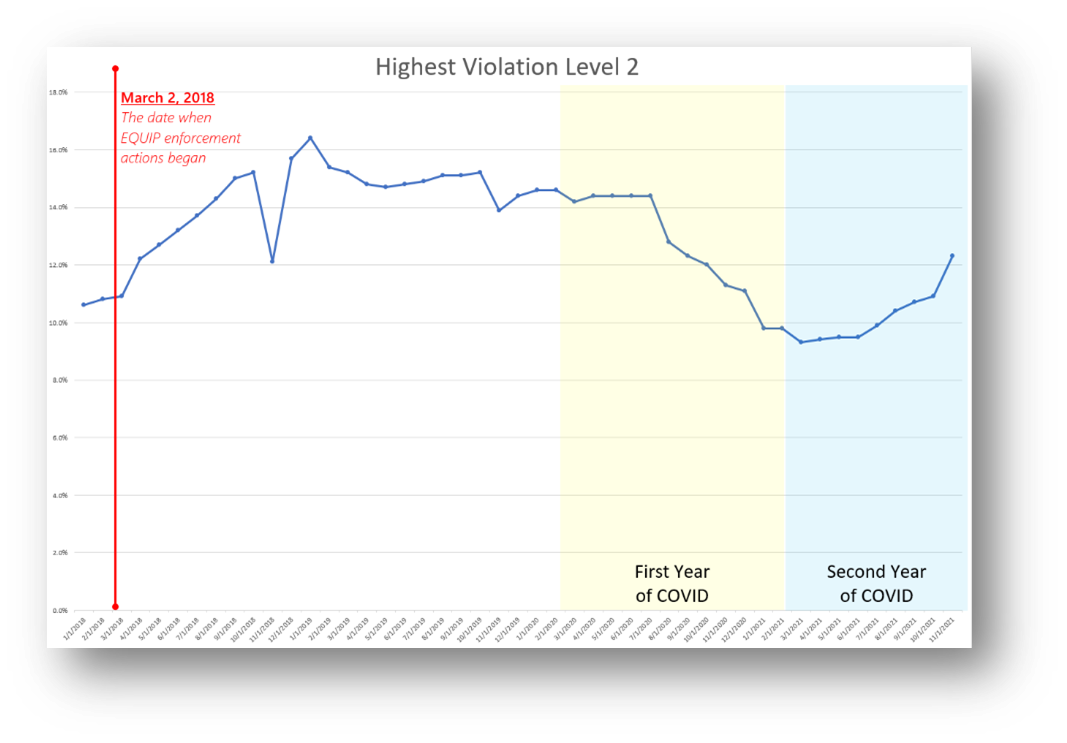
Prior to November 2015 the number of Level 2 violations was in a long, slow decline, as shown in the chart below[i]. It was evident during that period that the quality of mammography images was not improving, and even spurred the development of some novel image quality assessment software tools. For some unexplained reason, the downward trend reversed at that time.
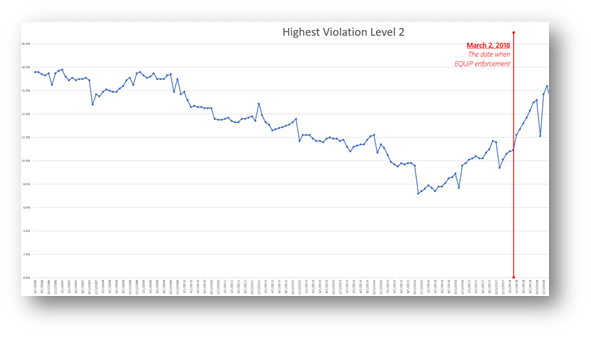
FDA began EQUIP inspections in March 2018. The rising trend in Level 2 violations since continued through the end of 2018. But then, as shown below, the trend reversed once again. Perhaps breast center managers (or MQSA inspectors) can respond to this blog and add their thoughts as to why that was the case.
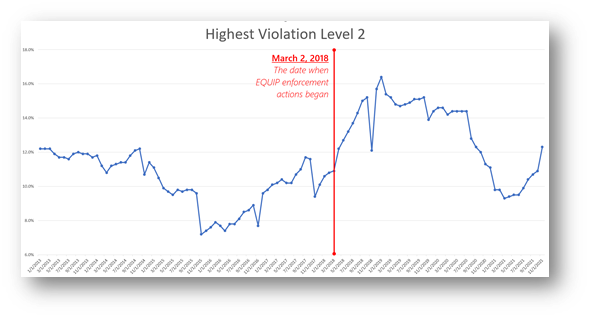
Ideally, of course, it was because breast centers, after a period of learning and training, got behind the principles of EQUIP and began improving their practices accordingly. I would like to think that’s the case. But then a quite awful thing happened – COVID.
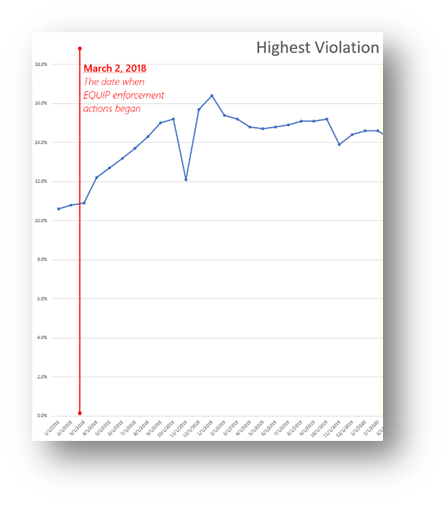
During the first year of COVID, the percentage of Level 2 violations dropped quickly (below). The reason for the drop is not clear. One could hope that it was due to facilities continuing to improve their understanding and compliance with EQUIP. Clearly the rate of Level 2 violations had stopped increasing following introduction of EQUIP. But the drop during COVID was significant.
However, in the second year of COVID the trend reversed quite sharply, with Level 2 violations increasing once again. Could this be due to more intense scrutiny by inspectors once we were used to masks and social distancing? Or something else?
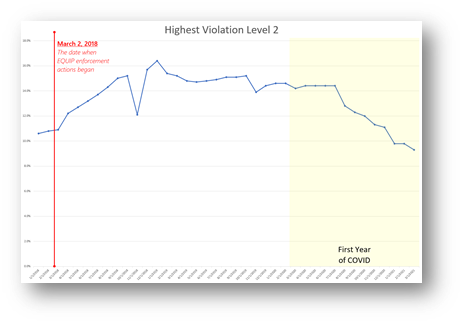
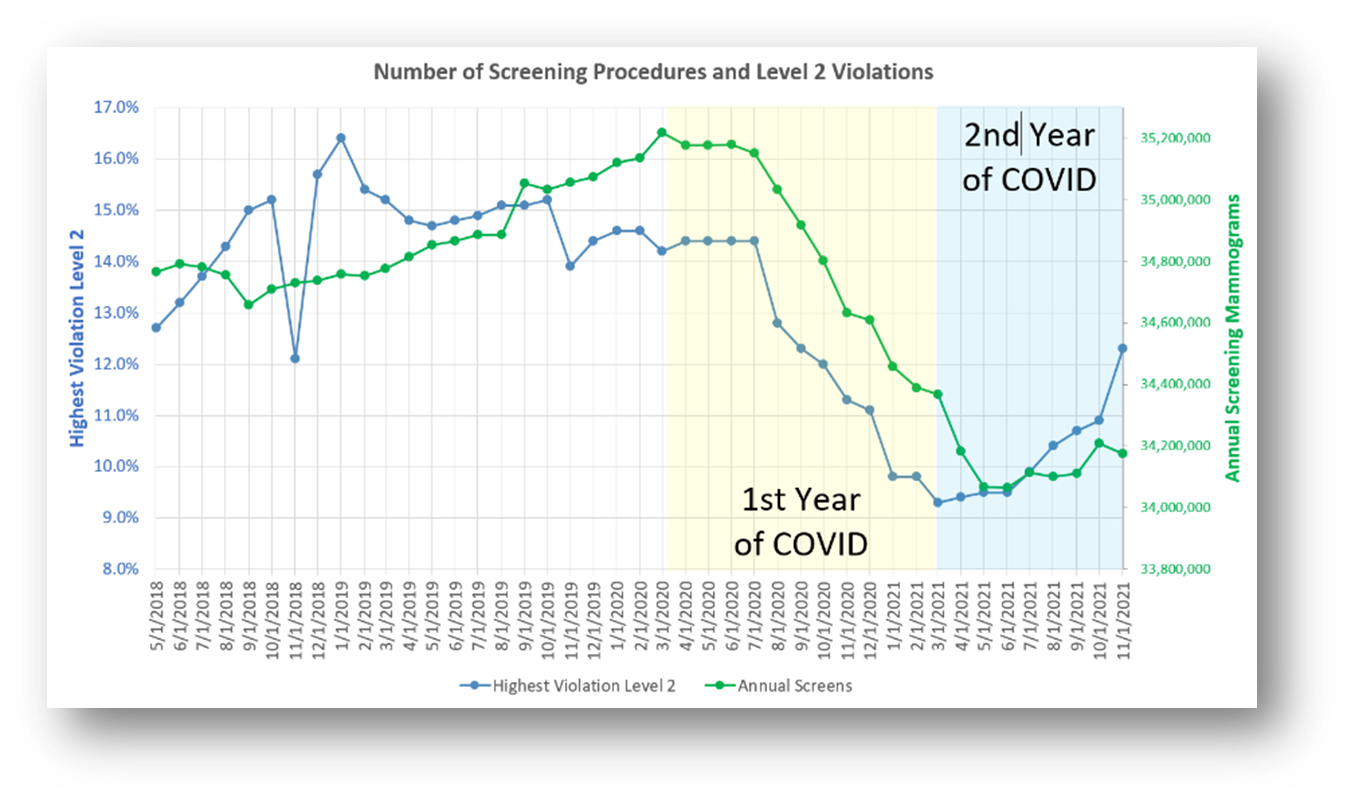
As curious as it may seem, the Level 2 violations track rather well with screening volumes, as is shown below. During the 1st year of COVID, screening volumes declined significantly, as has been well documented. As it so happens, the decline in screening volume seems to match the decline in Level 2 violations.
Does it that really make sense? Could there a causal relationship between one and the other, or is it just a coincidence?
Ideally, of course, it was because breast centers, after a period of learning and training, got behind the principles of EQUIP and began improving their practices accordingly. I would like to think that’s the case. But then a quite awful thing happened – COVID.
[i] Source: US FDA MQSA National Statistics. https://www.fda.gov/radiation-emitting-products/mqsa-insights/mqsa-national-statistics